Nagpur: India’s Bharat Biotech is all set to produce the world’s only malaria vaccine that has been approved by the World Health Organisation (WHO) and developed by pharma major GSK.
The launch of the vaccine in the market may take a couple of years for use. Earlier in January this year, Hyderabad-based vaccine major announced that it had entered a product transfer partnership with GSK for its malaria vaccine. According to a report, GSK would transfer RTS manufacturing technology to Bharat Biotech to produce the S antigen component of the vaccine and the license.
GSK will be making 15 million annual doses at not more than 5 per cent above the cost of production. However, Bharat Biotech is yet to comment on the development. As per a source, GSK is conducting the supplies from one of its plants and is planning to move entire production to Bharat Biotech by 2028.
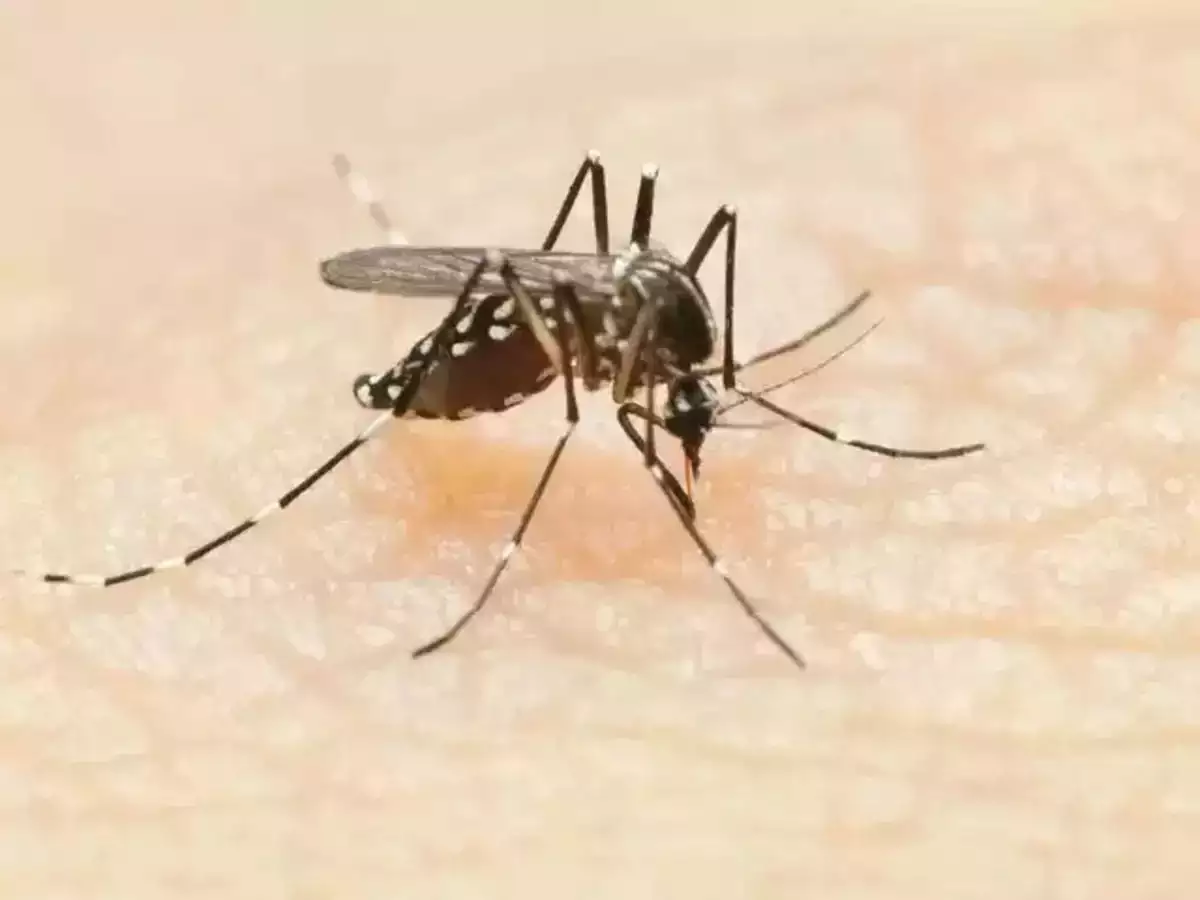
The World Health Organisation on Wednesday approved the RTS,S/AS01 malaria vaccine. It is the first vaccine against malaria which kills over 4,00,000 people every year, mostly in the African region.
This is considered to be a key turning point in the battle between man and mosquito which has been going on since decades. WHO Director-General Tedros Adhanom Ghebreyesus while addressing a press conference said, “This is a historic moment. The long-awaited malaria vaccine for children is a breakthrough for science, child health and malaria control… Using this vaccine on top of existing tools to prevent malaria could save tens of thousands of young lives each year.”