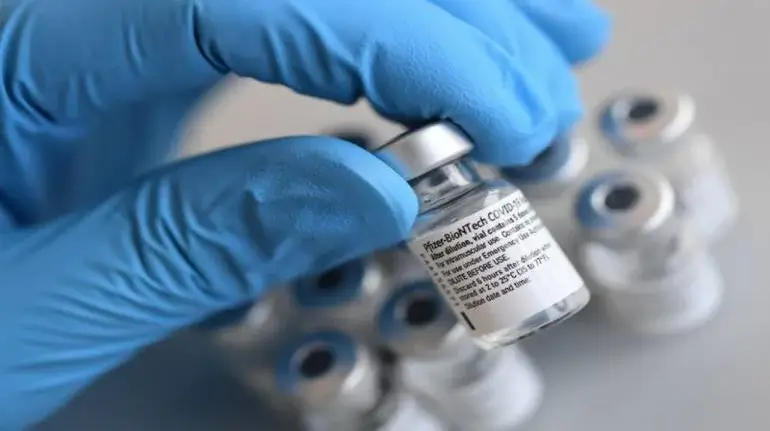
Nagpur: The US Food and Drug administration on Friday authorised emergency use of the Pfizer-BioNTech Covid-19 vaccine for children aged five to 11 years. After this development, the Pfizer-BioNTech has become the first coronavirus vaccine to get the FDA’s approval in the US.
The leading vaccine manufacturing firms—Pfizer and BioNTech said that the vaccine would be administered in a two doses regime with a gap of 21 days. The shot is said to be immediately available to the age group. However, the US Centers for Disease Control and Prevention is yet to advise on how the shot should be administered which will be decided after a meeting which is due on November 2 and 3.
The vaccine giant, Pfizer, said that it will begin shipping the paediatric vials to the pharmacies, paediatrician’s offices and other locations where the shots will be administered on Saturday.
The vaccine will be available to nearly 28 million children in America and comes as a relief as children have started to go back to school. Only a few other countries such as China, Chile, UAE have cleared the vaccine for the children.

Vaccine for children has been cleared in India too and the guidelines for the same will be issued shortly as per a government official. A government official said that several top experts in the field are being consulted to see how the Covid-19 vaccine among children can be introduced in the programme. “The guidelines on a list of specific comorbidities and other procedural clarifications should be out soon,” the official added.